X
Advancing research at ATSU
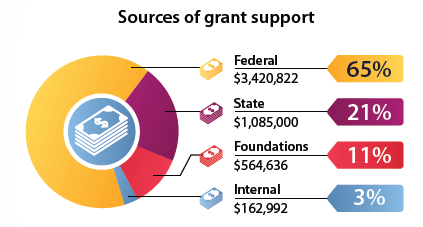
Sponsored Programs supports research at ATSU by facilitating funded grants, proposal development, and grant management compliance.Grants awards and sponsors
ATSU manages a diverse portfolio of research, service, and educational grant awards from many prestigious funding sources. Since 1985, ATSU has received over $65 million in U.S. Department of Health and Human Services (HHS) - Health Resources and Services Administration (HRSA) funding for primary care training initiatives and $29+ million from the National Institutes of Health, Substance Abuse & Mental Health Services Administration, Centers for Disease Control and Prevention, and other federal sources.