|
|
|
|
ANATOMY:
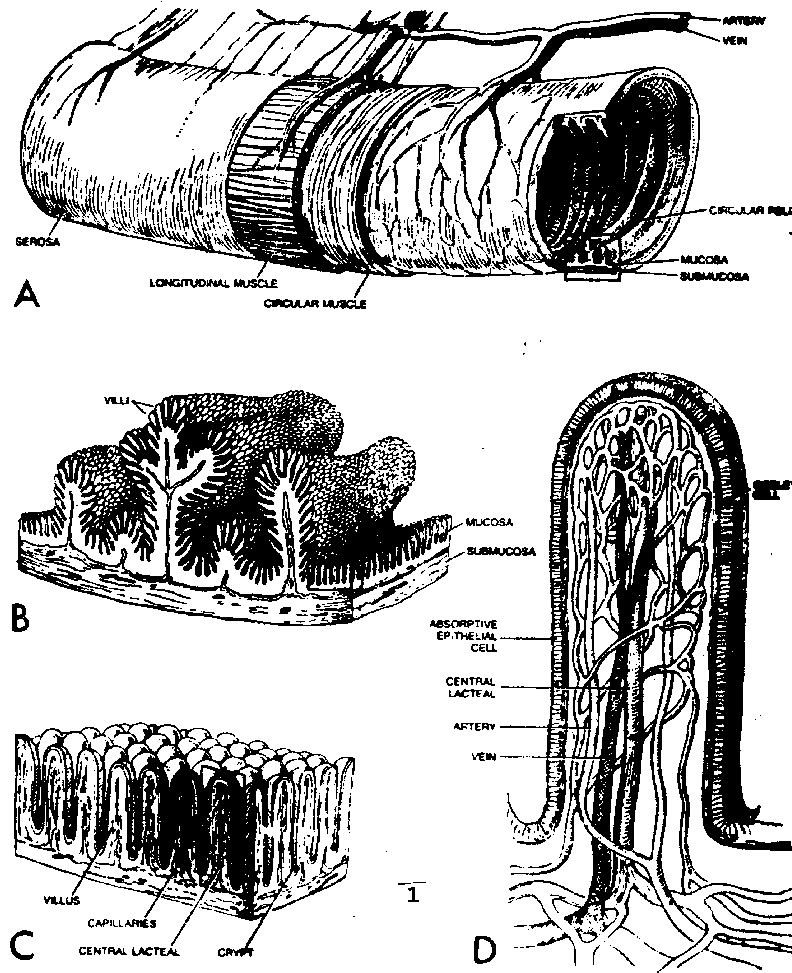
The small intestine of the human consists of three portions: duodenum (10 inches), jejunum (8 feet), and ileum (12 feet). The wall of the intestine is made up of mucosa and submucosa.

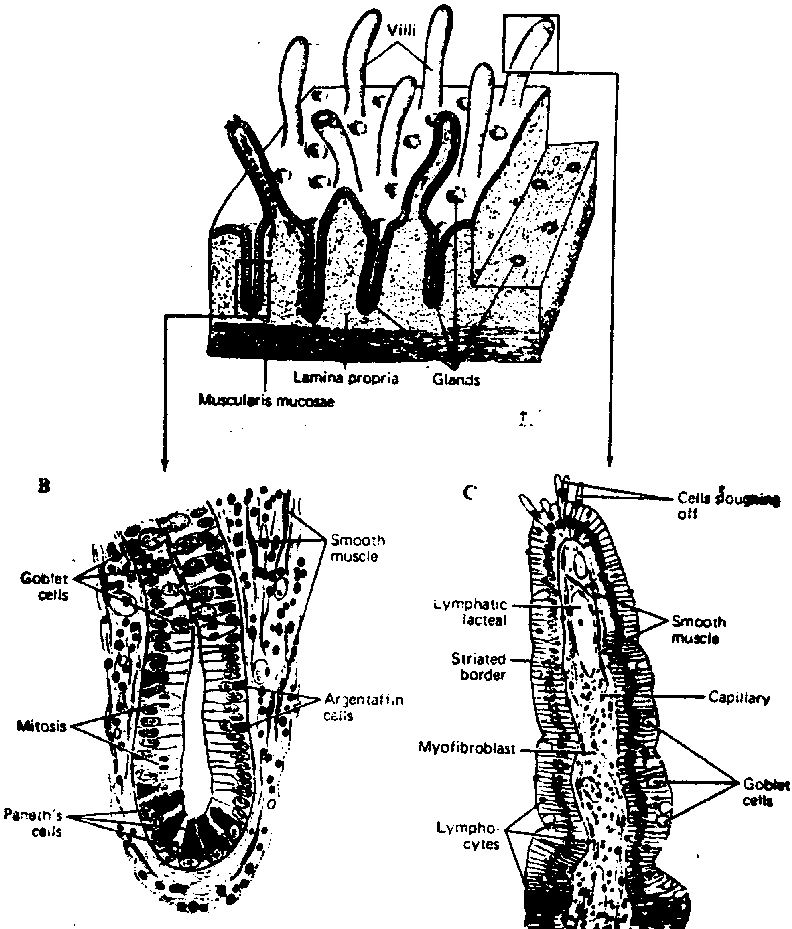
The intestinal epithelium is a continuous single layer of cells that covers the entire surface of the intestinal wall facing the intestinal lumen. It covers the villi, the area between the villi as well as the sides and bases of the crypts. The intestinal epithelium consists of 4 cell types: the columnarabsorptivecell (making up 90% of the total cells), the gobletcell (9.5% of total), the Paneth cell and the undifferentiated columnar cell.
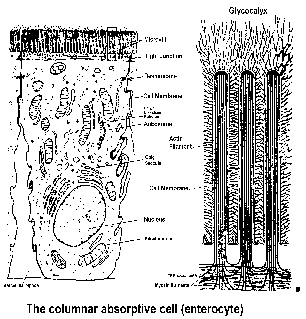
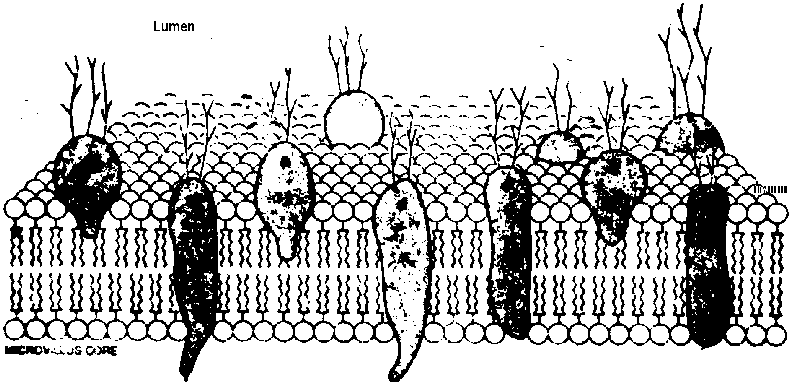
Covering the sides and tips of the villus is the columnar absorptive cell. This cell has, on its luminal surface, projections termed microvilli. These microvilli in turn, have projections of glycoprotein molecules which are termed the glycocalyx. This glycocalyx has enzymatic properties, i.e. it is a saccharidase, alkaline phosphatase and aminopeptidase. These glycoprotein enzymes have a hydrophobic end imbedded in the lipid of the cell membrane and a hydrophilic end projecting into the lumen. This hydrophilic end contains the substrate binding site. The function of the columnar absorptive cell is the absorption of water, minerals, amino acids and simple sugars.
The undifferentiated columnar cells occur at the base of the crypt where they are interspersed with the Paneth cells. This is the cell production site of the epithelium. These cells are in constant replication; they are the progenitor of the columnar absorptive cell, the goblet cell and Paneth cell.
Underlying the intestinal epithelium is the lamina propria. This is a compact stroma, composed mainly of reticular and elastin fibers. It has extensive beds of blood and lymph capillaries and it is into these that the absorbed food matter passes. Plasma cells and lymphocytic cells are especially numerous in this tissue, the latter often being amassed into solitary or aggregated lymph follicles (Peyer's patches). There is extensive trafficking of lymphocytes through the Peyer's patches. Thus, this presents a second line of immune defense against microbial invasion from the lumen of the intestine.
The muscularis mucosa is a thin bilaminar plexus of circular (inner)
and longitudinal (outer) smooth muscle fibers which clearly demarcates
the mucosa and submucosa. The muscularis mucosa enables alteration of the
focal conformation of the mucosa independent of other movements of the
digestive tract, increasing its contact with food.
The intestine absorbs both exogenous fluids and its own secretions. The secretions of the intestine and digestive glands are so great that, were they not resorbed, death from dehydration would result in 24 hours.
| Daily flux of gut water and sodium.
|
Gut Water | |
| ml/day | Na, mM/day | |
| Oral intake | 2,000 | 50-100 |
| Saliva | 1,000 | 50 |
| Gastric juice | 2,000 | 100 |
| Pancreatic juice | 2,000 | 200 |
| Bile | 1,000 | 150 |
| Intestine secretion | 6,200 | 840 |
| Sum | 14,200 | 1,440 |
| Transit ileum to colon | 1,500 | 200 |
| Fecal | 100-160 | 5 |
Only a few of the absorptive processes are regulated. The intestine indiscriminately absorbs water, the major electrolytes, and the products of digestion of foodstuffs, but absorption of calcium and iron is adjusted to the body's needs. Three properties of the intestinal mucosa determine its handling of water and electrolytes:
3. Cl- - HCO3- exchange. In the ileum,
but not in the jejunum, there is equal exchange of Cl- from
the luminal fluid for HCO3-, from the cell interior
across the apical membrane of the epithelial cells. Cl- entering
the cell is extruded across the basolateral border into interstitial fluid.
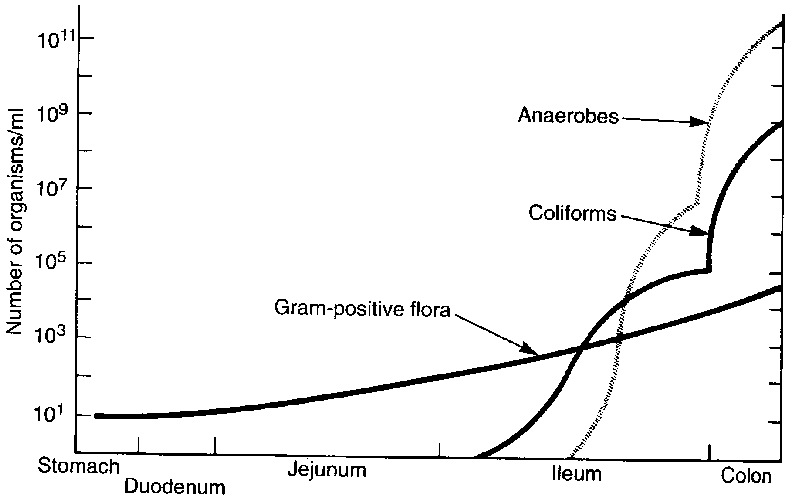
The mucous membrane of the large intestine does differ from that of the small intestine in several aspects:
2. The intestinal crypts are larger, more numerous and more densely packed.
3. One-fourth of the epithelial cells are goblet cells (vs. one-tenth in the small intestine). Thus the large intestine is well lubricated with a heavy mucous.
4. There are no Paneth cells.
Water moves passively with Na+ and Cl-, but neither
the movement of these ions nor of water is unidirectional or isotonic.
Na+ and its accompanying anion and water diffuse fairly easily
back into the lumen, but the net flow is usually in the direction of active
absorptions into the portal blood. This is usually 4 times as rapid as
back diffusion into the lumen.
Davenport, H.W. 1982. Physiology of the Digestive Tract, 5th Edition. Year Book Medical Publishers, Inc. Chicago, Illinois.
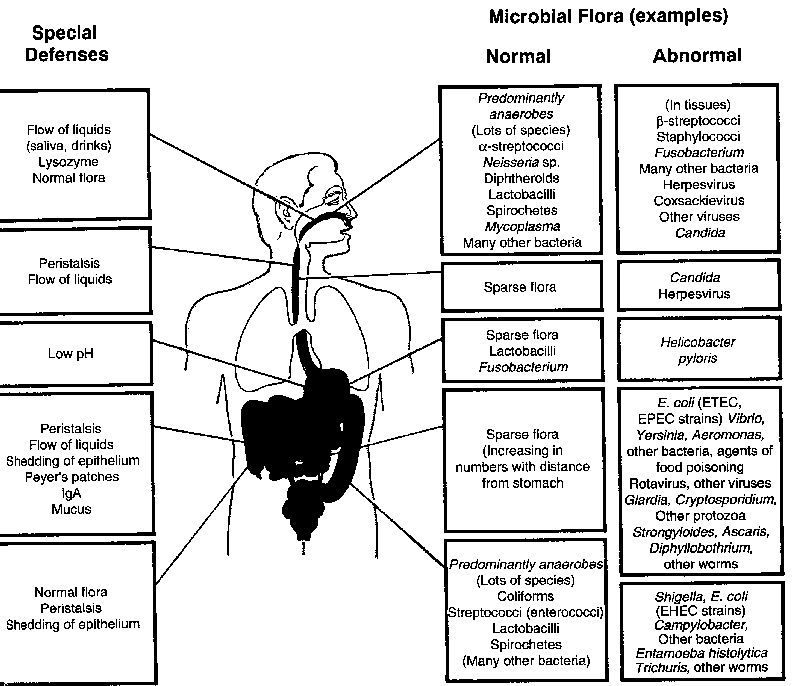
Consider the gastrointestinal tract as a tube through the center of the body extending from the oral cavity to the anus. The walls of this tube serve as the interface between the external environment and the body. Through this tube passes all of the liquid and solid material we ingest. Carried with the ingested material are bacteria which tend to colonize those parts of the tube that offer a suitable environment for growth, establishing a "normal" flora for each part of the tube.
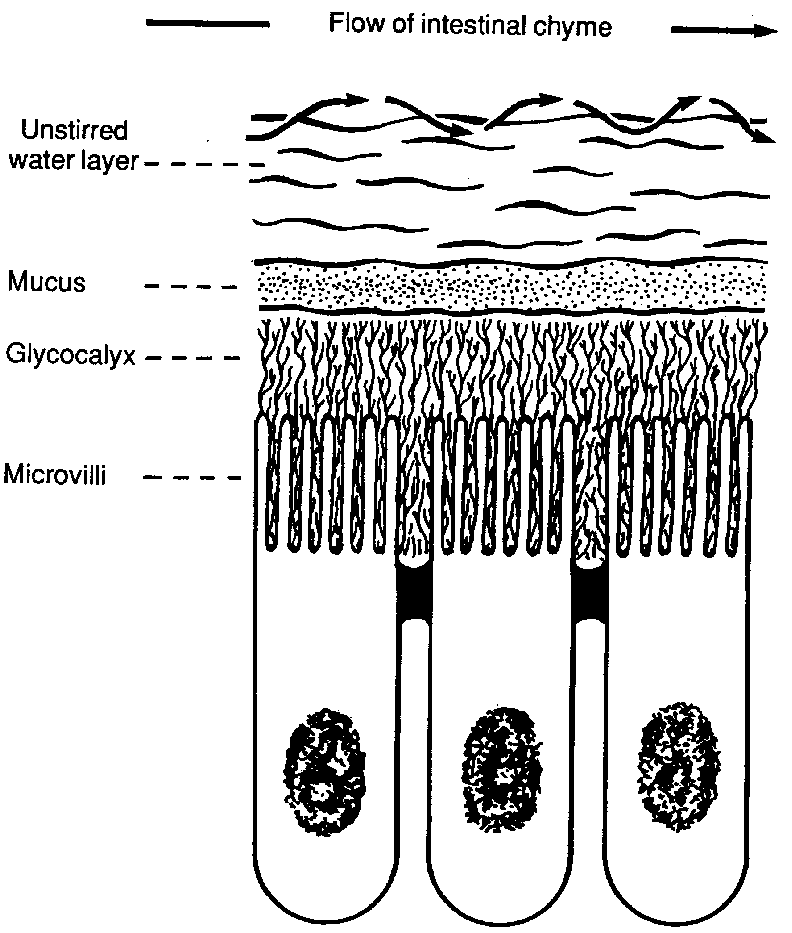
B. The glycocalyx, a glycoprotein and polysaccharide layer that covers
the surface of the epithelial cells. This presents a thick, relative to
the size of bacteria, physical barrier as well as a chemical trap that
binds microorganisms.
D. Acidity of the stomach. The normal pH of the stomach is less than 4. This acidity spills into the small intestine establishing a pH gradient that prevents most bacteria from colonizing the stomach, duodenum, jejunum and upper half of the ileum. Because of this, the majority of ingested pathogens never reach the intestinal tract. Over 99.9% of ingested bacteria are killed after 30 minutes exposure to stomach acidity. Alteration of the acid barrier of the stomach by disease, surgery, drugs or antacids increases the survival of pathogens across this organ and may lead to microbial infection downstream. For example, the inoculum of Vibrio cholerae required to cause disease is 108 organisms. If gastric acidity is neutralized by 2 gm of sodium bicarbonate, only 104 ingested organisms are required to cause disease.
E. Bile. Bile solubilizes lipids; it thus inactivates those organism having a lipid envelope. All enveloped viruses and many bacteria are thus prevented from growing in areas of high bile salts. Obstruction of the flow of bile due, for example, to gallstones has two effects: downstream from the blockage and into the intestine non-normal flora (i.e., organisms with an outer lipid membrane) can proliferate and cause disease and upstream from the block bile salts accumulate and initiate a cycle of inflammation and damage to the gallbladder wall which often becomes a site of infection (cholecystitis)
F. IgA. Secretary IgA helps prevent colonization by certain species of bacteria.
H. While blood cells, especially neutrophils, have their final stopping point in the intestine. They play an undetermined role in controlling pathogens and maintaining the balance of normal flora.
I. Gut motility. Peristalsis contributes to the health of the gut by:
2. Maintaining appropriate dilution of indigenous enteric microflora
3. Ridding the host of pathogenic microorganism by hindering adherence
of micro-organisms to receptors in the epithelial wall.
K. Normal flora. Of the normal microflora, 99.9% are anaerobes, mainly
members of the genera Bacteroides, Clostridium, and Peptostreptococcus.
The remaining organisms are aerobes or facultative cells of the genera
Escherichia,
Proteus
and Pseudomonas as well as other less numerous species. These normal
non-pathogenic flora compete with potential pathogens for nutrients and
intestinal receptor sites, thus keeping them from causing disease.
B. Antibiotic therapy which destroys the normal flora and thus reduces competition that pathogens are normally subjected to.
C. Glucosteroid therapy which reduces the immune reaction.
E. Radiation therapy which affects immunity and sometimes upsets the balance of normal flora and intestinal epithelium integrity.
F. Ingestion of pre-formed toxins with food and/or water.
G. Ingestion of microorganisms which produce toxins/enzymes/ immune suppression factors in situ.
H. Anatomic alterations. Obstructions to the flow of liquids remove
one of the most powerful defensive mechanisms of the gastrointestinal tract.
Thus, stones in the gallbladder that impede the flow of bile predispose
the biliary tree to infections. The presence of large diverticuli or the
surgical formation of intestinal "blind loops" create sites with reduced
flow of intestinal contents, leading to bacterial overgrowth and metabolic
derangements.
2. Is there any history of family members suffering gastrointestinal symptoms?
3. Is any family member on medication for gastrointestinal symptoms?
2. Sex
3. Race
4. Recent travels
5. Chief complaint (pain)
b. Frequency
d. Severity (mild, moderate, severe)
e. Duration
f. Type of pain
(2) Dull
(3) Shooting
(4) Cramplike
(5) Stabbing
(6) Gnawing
(7) Burning
7. Presence of gas
8. What measures alleviate symptoms?
b. Vomiting
c. Expelling gas
d. Bowel movement
e. Rest
f. Change in position (e.g. sitting up or walking around) - hiatus hernia
12. Previous invasive procedures
13. Previous roentgenographic examinations
14. Appetite
15. Food intolerance
16. Nausea
17. Emesis (vomiting)
18. Regurgitation
19. Hematemesis (vomiting of blood)
20. Eructation (oral ejection of gas or air from stomach)
21. Dyspepsia (indigestion)
22. Jaundice
23. Ascites (fluid in the peritoneal cavity)
24. Frequency of bowel movements
25. Diarrhea
26. Flatulence
27. Melena (darkening of feces by blood pigments)
28. Hematochezia (passage of bloody stools)
2. Stria
4. Pulsations
5. Anterior hernias
2. Bruits - especially femoral and renal (an abnormal sound or murmur)
3. Liver and splenic friction rub
2. Dullness
3. Liver span
4. Spleen span
5. Shifting dullness
2. Rigidity
3. Pulsating mass
4. Fluid wave
5. Hepatomegaly
6. Splenomegaly
7. Hepatojuguloreflux
8. Prostate enlargement
2. Microscopic examination
b. Sudan stain for fat globules (large fat globules indicates malabsorption)
c. Eosin stain (stains undigested muscle fibers, indicating pancreatic insufficiency and maldigestion)
d. pH (acidic pH indicates lactose intolerance in children) - normal pH is greater than 7.
e. Copper sulfate reaction - presence of reducing sugars indicates carbohydrate malabsorption.
f. Occult blood test
g. Culture for enteric pathogens
C. Serological tests (e.g., typhoid fever, amebiasis)
D. Toxin assays - Rabbit loop test, Sereny test, adrenal cell assay
F. Pathological examination
|
|
|
|