General Goal: To know the major causes of this disease progression, understand the basic processes that cause the progression from SIRS to septic shock, and describe the basic treatment plan in caring for these patients.
Specific Educational Objectives: The student should be able to:
1. recite the most likely causes of sepsis based on the knowledge of the initial site of infection and where these organisms usually come from (sources of infection).
2. recite the most common causes of anaerobic sepsis and pediatric sepsis.
3. recite the factors that increase the risk of a patient getting sepsis and the patient types most like to get sepsis.
4. recite the major sites of infection that can lead to sepsis.
5. describe the sequence of events that lead to septic shock (know the microbial triggers and the host mediators that led to septic shock). A basic understanding of what types of shock are caused by sepsis.
6. describe the differences between the following: SIRS, sepsis, severe sepsis, septic shock, and MODS.
7. Recite the 3 treatment priorities and understand their importance.
Reading: F.S. Southwick, Infectious Diseases in 30 Days, Chapter 2: The Sepsis Syndrome, 2003, McGraw Hill.
Lecture: Dr. Neal R. Chamberlain
Resources:
eMedicine
Online: Septic Shock, by J Stephan Stapczynski, MD, Chair, Associate
Professor, Department of
Emergency Medicine, University of Kentucky Chandler Medical Center
(last revised 0/01/00; http://www.emedicine.com/cgi-bin/foxweb.exe/showsection@d:/em/ga?book=emerg&topicid=533).
Balk, R.A., Casey, L.C., Sepsis and Septic Shock. Critical Care Clinics. April 2000.
Angus DC, Linde-Zwirble WT, Lidicker J, et al.: Epidemiology of severe sepsis in the United States: analysis of incidence, outcome and associated costs of care. Crit Care Med 2001, 29:1303-1310
Enterococcus sp.
Pediatric septicemia - S. pneumoniae, Neisseria meningitidis, or S. aureus usually causes sepsis in the child. Sepsis due to H. influenzae was very common however since the introduction of the Hib vaccine, invasive H. influenzae infections have virtually disappeared. Other causes include E. coli, S. agalactiae (Group B Strep), Klebsiella sp. and Enterobacter sp. Sepsis is the 7th leading cause of death in children 1-4 years of age and is the 9th leading cause of death in children 5-14 years of age (2003).
Sepsis in the neonate is most likely to be caused by S. agalactiae (group B strep. is the leading cause of neonatal sepsis), E. coli, Klebsiella sp. or Enterobacter sp.
Bloodstream infections caused by Pseudomonas aeruginosa, Candida albicans, or multidrug resistant Enterococcus faecium is associated with increased mortality.
Fungi, viruses (HIV etc.), and protozoa can also cause septic shock. Less common than bacterial causes.
|
|
|||||
|
|
|
|
|
|
|
| Major Community Acquired Pathogens | Streptococcus pneumoniae
Haemophilus influenzae Legionella sp. Chlamydia pneumoniae |
Escherichia coli
Bacteroides fragilis |
Streptococcus pyogenes
Staphylococcus aureus Clostridium sp. Polymicrobial infections
|
Escherichia coli
Klebsiella sp. Enterobacter sp. Proteus sp. |
Streptococcus pneumoniae
Neiserria meningitidis Listeria monocytogenes Escherichia coli Haemophilus influenzae |
| Major Nosocomial pathogens | Aerobic gram negative bacilli | Aerobic gram negative bacilli
Anaerobes Candida sp. |
Staphylococcus aureus
Aerobic gram negative bacilli |
Aerobic gram negative bacilli
Enterococcus sp. |
Pseudomonas aeruginosa
Escherichia coli Klebsiella sp. Staphylococcus sp. |
Special Concerns:
Elderly patients are more susceptible to sepsis, have less physiologic reserve to tolerate the insult from infection, and are more likely to have underlying diseases, all of which adversely impact survival. In addition, elderly patients are more likely to have atypical or nonspecific presentations when septic.
S. pneumoniae, Neisseria meningitidis, or S aureus usually causes sepsis in the child. Sepsis due to H. influenzae was very common however since the introduction of the Hib vaccine, invasive H. influenzae infections have virtually disappeared.
Sepsis and septic shock in the immunocompromised patient is associated
with a wide variety of bacteria and fungi.
Gram-negative septic shock: comprises 1/2 of total cases of sepsis, 115,000 deaths/year. As a group gram negative bacteria cause more deaths due to sepsis.
Gram-positive septic shock: more gram positive cases of septic shock are seen due to the increased incidence in pneumonia and in the use of intravascular devices, 1/2 of cases sepsis.
Factors contributing to the increasing incidence of sepsis:
The source of the infection is an important determinant of clinical outcome. Severe sepsis is most likely to occur in patients with nosocomial pneumonia. Patients with intra-abdominal infection and polymicrobial bacteremia or postoperative wound infections and bacteremia are at significant risk for severe sepsis. Bacteremia associated with intravascular catheters or indwelling urinary catheters carries a lower risk of developing septic shock.
Microbial triggers of disease:
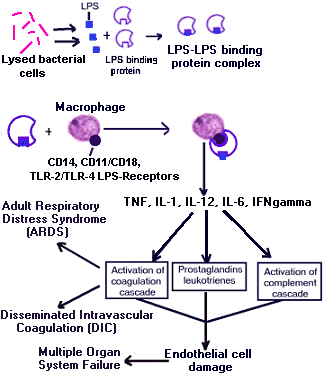
This is a very complex sequence of events and much work still needs to be done to completely understand how a patient goes from SIRS to septic shock. Patients with septic shock have a biphasic immunological response. Initially they manifest an overwhelming inflammatory response to the infection. This is most likely due to the pro-inflammatory cytokines Tumor Necrosis Factor (TNF), IL-1, IL-12, Interferon gamma (IFNgamma), and IL-6.
The body then regulates this response by producing anti-inflammatory cytokines (IL-10), soluble inhibitors [TNF receptors, IL-1 receptor type II, and IL-1RA (an inactive form of IL-1)]. Which is manifested in the patient by a period of immunodepression. Persistence of this hyporesponsiveness is associated with increased risk of nosocomial infection and death.
This systemic inflammatory cascade is initiated by various bacterial products. These bacterial products (gram-negative bacteria= endotoxin, formyl peptides, exotoxins, and proteases, gram-positive bacteria= exotoxins, superantigens (toxic shock syndrome toxin (TSST), streptococcal pyrogenic exotoxin A (SpeA)), enterotoxins, hemolysins, peptidoglycans, and lipotechoic acid, and fungal cell wall material) bind to cell receptors on the host's macrophages and activate regulatory proteins [Nuclear Factor Kappa B (NFkB)]. Endotoxin activates the regulatory proteins by interacting with several receptors. The CD receptors pool the LPS-LPS binding protein complex on the surface of the cell and then the TLR receptors translate the signal into the cells.

Then these primary and secondary mediators cause the activation of the coagulation cascade, the complement cascade and the production of prostaglandins and leukotrienes. Clots lodge in the blood vessels which lowers profusion of the organs and can lead to multiple organ system failure. In time this activation of the coagulation cascade depletes the patient's ability to make clot resulting in DIC and ARDS.
The cumulative effect of this cascade is an unbalanced state, with inflammation dominant over antiinflammation and coagulation dominant over fibrinolysis. Microvascular thrombosis, hypoperfusion, ischemia, and tissue injury result. Severe sepsis, shock, and multiple organ dysfunction may occur, leading to death.
Symptoms of sepsis are usually nonspecific and include fever, chills, and constitutional symptoms of fatigue, malaise, anxiety, or confusion. These symptoms are not pathognomonic for infection and may be seen in a wide variety of noninfectious inflammatory conditions. They may be absent in serious infections, especially in elderly individuals.
The following is the 1992 Consensus Conference's definitions for diagnosis of SIRS to MODS.
CONSENSUS CONFERENCE DEFINITIONS*
Systemic Inflammatory Response Syndrome (SIRS): Patient presents with two or more of the following criteria.
Severe Sepsis: Sepsis associated with organ dysfunction, hypoperfusion abnormalities, OR hypotension. Hypoperfusion abnormalities include but are not limited to:
Organ Dysfunctions associated with Severe Sepsis and Septic Shock:
Lungs: early fall in arterial PO2, Acute Respiratory Distress Syndrome (ARDS): capillary-leakage into alveoli; tachypnea, hyperpnea
Kidneys (acute renal failure): oliguria, anuria, azotemia, proteinuria
Liver- elevated levels of serum bilirubin, alkaline phosphatase, cholestatic jaundice
Digestive tract- nausea, vomiting, diarrhea and ileus
Skin - ecthyma gangrenosum (think Pseudomonas aeruginosa in neutropenic patients), Petechia or purpura (think Neisseria meningitidis or Rickettsia rickettsia (if evidence of tick bite)), Hemorrhage or bullous lesions in patient who has eaten raw oysters (Vibrio vulnificus), generalized erythroderma (Toxic Shock Syndrome= Staphylococcus aureus or Streptococcus pyogenes)
Heart- cardiac output is initially normal or elevated,
Brain - confusion
Multiple Organ Dysfunction Syndrome (MODS): Presence of altered organ function in an acutely ill patient such that homeostasis cannot be maintained without intervention.
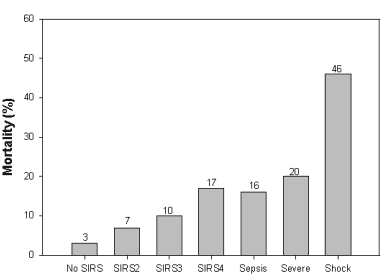
Mortality increases with increase in number of SIRS symptoms and
in severity of the disease process. (From Rangel-Frausto, M., Pttet,
D., Costigan, M., et. al. The natural history of the systemic inflammatory
response syndrome (SIRS). JAMA 273:117-123, 1995)
Complications:
Adult respiratory distress syndrome (ARDS)The reported incidence of these complications in SIRS and sepsis in different studies is about 19% for CNS dysfunction, 2-8% for ARDS, 12% for liver failure, 9-23% for ARF, and 8-18% for DIC.
Disseminated Intravascular Coagulation (DIC)
Acute Renal failure (ARF)
Intestinal bleeding
Liver failure
Central Nervous system dysfunction
Heart failure
Death
In septic shock, ARDS has been observed in about 18%, DIC in about 38%,
and renal failure in about 50%.
The diagnosis of sepsis requires a high index of suspicion, the taking of an EXCELLENT history, physical examination, appropriate laboratory tests, and a close follow-up of hemodynamic status.
History
Helps in determining if the infection was community or nosocomially
acquired and if the patient is immunocompromised. Important details include
exposure to animals, travel, tick bites, occupational hazards, alcohol
use, seizures, loss of consciousness, medications, and underlying diseases
that may predispose the patient to specific infectious agents. Some clues
to a septic event include:
Physical Examination
A thorough physical exam is essential. In all neutropenic patients
and in patients with as suspected pelvic infection the physical exam should
include rectal, pelvic, and genital examinations. Such exams may reveal
rectal, perirectal, and/or perineal abscesses, pelvic inflammatory disease
and/or abscesses, or prostatitis.
Laboratory data
Respiratory alkalosis signals impending shock that is reversible with fluid resuscitation. Metabolic acidosis can develop just prior to hypotension or can occur at the same time. Metabolic acidosis can signal the beginning of the end for the patient. Treatment should be instituted before metabolic acidosis begins.
Recent studies indicate that procalcitonin (PCT) is a good nonspecific marker for differentiating systemic bacterial inflammatory responses from nonbacterial systemic inflammatory responses. One study (Ann Rheum Dis. 2003;62:337-340) suggests that "patients with fever or inflammatory syndrome who have PCT levels greater than 1.2 ng/ml, we consider that bacterial infection should be sought and antibiotic treatment started even before the results of the bacteriological investigations are obtained. This approach is even more strongly recommended in patients with inflammatory disease given immunosuppressive treatment. In contrast, once tuberculosis and mycobacterial infection have been ruled out, normal PCT levels do not argue in favor of bacterial infection."
Other tests tests include CBC with differential count, C-reactive protein, urinalysis, coagulation profile, glucose, blood urea, nitrogen, creatinine, electrolytes, liver function tests, lactic acid level, arterial blood gas, electrocardiogram, and a chest X-ray. Cultures of blood, sputum, urine, and other obviously infected sites should be performed. Gram's stain of normally sterile sites (blood, CSF, articular fluid, pleural space) by aspiration. At least 2 sets (some believe 3) of blood cultures should be obtained over a 24hr period. Sample volume: there is often less than 1 bacterium/ml in adults (higher in children). Draw 10-20 ml per sampling in adults (1-5 ml in children) and inoculate both trypticase soy broth and thioglycolate broth. Sample time: for intermittent fever spikes, the bacteremia is most prominent 0.5 hr before the spike. If antibiotic therapy has been initiated, some antibiotics can be deactivated in the clinical lab.
Depending on the patient's clinical status and associated risks other studies could include abdominal X-ray, CAT scans, MRI, 2D echocardiograms, and/or lumbar puncture.
Other laboratory findings:
EARLY SEPSIS; leukocytosis with left shift, thrombocytopenia, hyperbilirubinemia, and proteinuria. Leukopenia may occur. Neutrophils may contain toxic granulations, Dohle bodies, or cytoplasmic vacuoles. Hyperventilation commonly induces respiratory alkalosis. Hypoxemia correctable with oxygen. Diabetics can develop hyperglycemia. Serum lipids are elevated.
LATER ON: Thrombocytopenia worsens with prolongation of thrombin time, decreased fibrinogen, and presence of D-dimers suggesting DIC. Azotemia, and hyperbilirubinemia are more prominent. Aminotranferases (liver enzymes) become elevated. When respiratory muscles fatigue the accumulation of serum lactate occurs. Metabolic acidosis (increased anion gap) supervenes the respiratory alkalosis. Hypoxemia not correctable even with 100% oxygen. Diabetic hyperglycemia can precipitate ketoacidosis worsening the hypotension.
The immediate concern for patients with severe sepsis is reversal of life-threatening abnormalities (ABCs: airway, breathing, circulation). Altered mental status or depressed level of consciousness secondary to sepsis may require immediate protection of the patient's airway. Intubation may also be necessary to deliver higher oxygen concentrations. Mechanical ventilation may help lower oxygen consumption by the respiratory muscles and increase oxygen availibility for other tissues. Circulation may be compromised and significant decreases in blood pressure may require aggressive combined empiric therapy with fluids (with crystalloids or colloids) and inotropes/vasopressors (dopamine, dobutamine, phenylephrine, epinephrine, or norepinephrine). In severe sepsis monitoring of the circulation may be necessary. Normal CVP (central venous pressure) is 10-15 cm of 0.9% NaCl; normal PAW (pulmonary arterial wedge pressure) is 14-18 mm Hg; maintain adequate plasma volume with fluid infusion.
Prompt institution of empiric treatment with antimicrobials is essential. The early institution of antimicrobials has been shown to decrease the development of shock and to lower the mortality rate. After the appropriate samples are obtained from the patient a regimen of antimicrobials with broad spectrum of activity is needed. This is because antimicrobial therapy is almost always instituted before the organisms causing the sepsis are identified.
The drugs used depends on the source of the sepsis*.
Single drug regimens are usually only indicated when the organism causing sepsis has been identified and antibiotic sensitivity testing has revealed which antimicrobials the organism is sensitive to.
Eli Lilly and Company announced in 2001 the results of a Phase III clinical trial that demonstrated drotrecogin alfa (recombinant human activated protein C, Xigris used to be called Zovant) could reduce the relative risk of death from sepsis with associated acute organ dysfunction (known as severe sepsis) by 19.4 percent. These patients had Acute Physiology and Chronic Health Evaluation II Scores (APACHE II) of 25-53. To treat with this protein the patient should have an APACHE II score of between 25 and 53. Please note that patients treated with this protein also are at an increased risk of bleeding. This risk is highest during infusion of the protein.It is the first agent approved by the FDA effective in the treatment of severe sepsis proven to reduce mortality. Activated Protein C (Xigris) mediates many actions of body homeostasis. It is a potent agent for the:
- suppression of inflammation [a. directly suppresses monocyte production of nuclear factor-kB (NF-kB, b. inhibits thrombin generation which is proinflammatory, c. minimizes the expression of E-selectin on endothelial walls, producing dose-dependent inhibition of leukocyte adhesion at the site of infection. Reduced leukocyte/endothelial interaction down regulates oxygen radical release, decreasing vascular damage.],
- prevention of microvascular coagulation [a. proteolytic inactivation of Factors Va and VIIIa preventing thrombin formation.]
- reversal of impaired fibrinolysis [a. binds with plasminogen activator inhibitor-1 (PAI-1), causing inactivation of PAI-1. This in turn reduces the inhibition of tissue plasminogen activator (t-PA), allowing t-PA to stimulate fibrinolysis. Fibrin is broken down, and microcirculation is restored.].
Use trimethoprim-sulfamethoxazole prophylactically in leukemic children.
Use topical silver nitrate, silver sulfadiazine, or sulfamylon prophylactically in burn patients.
Apply polymyxin spray to the posterior pharynx to prevent nosocomial Gram-negative pneumonia.
Sterilization of the bowel aerobic flora with polymyxin or gentamycin with vancomycin and nystatin were effective in reducing Gram-negative sepsis in neutropenic patients.
Protective environments for patients at risk have not been to successful because most infections have endogenous origins.
To protect neonates from Group B strep sepsis obtain vaginal/rectal
swab samples at 35 to 37 weeks' gestation. Culture for Streptococcus
agalactiae (leading cause of neonatal sepsis). If positive for Group
B strep then give the mother intrapartum penicillin This will lower Group
B strep infections by about 78%.
Revised 8/24/04
Send comments and email to Neal R. Chamberlain, Ph.D., nchamberlain@atsu.edu
©2004 Neal R. Chamberlain, Ph.D., All rights reserved.