Previous Lecture | Syllabus | Next Lecture
![]()

2.
To differentiate the rickettsia, chlamydia and mycoplasma on the basis
of cell structure, metabolism, genetic
characteristics, pathogenicity, routes of disease transmission and methods
of diagnosis.
Specific educational objectives (terms and concepts upon which you will be tested)
1. To define the following terms and concepts:
The rickettsia are bacteria which are obligate intracellular parasites. They are considered a separate group of bacteria because they have the common feature of being spread by arthropod vectors (lice, fleas, mites and ticks). The cells are extremely small (0.25 u in diameter) rod-shaped, coccoid and often pleomorphic microorganisms which have typical bacterial cell walls, no flagella, are gram-negative and multiply via binary fission only inside host cells. They occur singly, in pairs, or in strands. Most species are found only in the cytoplasm of host cells, but those which cause spotted fevers multiply in nuclei as well as in cytoplasm. In the laboratory, they may be cultivated in living tissues such as embryonated chicken eggs or vertebrate cell cultures.
The family Rickettsiaceae is taxonomically divided into three genera:
2.
Ehrlichia
(2 species) - obligate intracellular parasites which do not multiply within
vacuoles but do parasitize
white blood cells.
3. Coxiella (1 species)--obligate intracellular parasite which grows preferentially in vacuoles of host cells.
4. Baartonella (3 species)--intracellular parasite which attacks the red blood cell.
The structure of the typical rickettsia is very similar to that of Gram-negative bacteria. The typical envelope consists of three major layers: an innermost cytoplasmic membrane, a thin electron dense rigid cell wall and an outer layer. The outer layer resembles typical membranes in its chemical composition and its trilaminar appearance. The cell wall is chemically similar to that of Gram-negative bacteria in that it contains diaminopimelic acid and lacks teichoic acid. Intracytoplasmic invaginations of the plasma membrane (mesosomes) and ribosomes are also seen. There are no discrete nuclear structures.
Metabolism
In dilute buffered salt solutions, isolated rickettsia are unstable, losing both metabolic activity and infectivity for animal cells. If, however, the medium is enriched with potassium, serum albumin and sucrose, the isolated organisms can survive for many hours. If ATP is added to the solution, the organisms metabolize and consume oxygen. The basis for the obligate parasitism of these cells is that they require the rich cytoplasm to stabilize an unusually permeable cell membrane.
The rickettsia have many of the metabolic capabilities of bacteria, but require an exogenous supply of cofactors to express these capabilities. The response to exogenous cofactors implies an unusually permeable cytoplasmic membrane.
Growth and Multiplication
Rickettsia normally multiply by transverse binary fission. Under poor nutritional conditions, the rickettsia cease dividing and grow into long filamentous forms, which subsequently undergo rapid and multiple division into the typical short rod forms when fresh nutrient is added. Immediately after division, the rickettsia engage in extensive movements through the cytoplasm of the cell. C. burnetii differs from other rickettsia in that it is enclosed in a persistent vacuole during growth and division. Six to ten daughter cells will form within a host cell before the cell ruptures and releases them.
Pathogenicity
In their arthropod vectors, the rickettsia multiply in the epithelium of the intestinal tract; they are excreted in the feces, but occasionally gain access to the arthropods salivary glands. They are transmitted to man, via the arthropod saliva, through a bite. In their mammalian host, they are found principally in the endothelium of the small blood vessels, particularly in those of the brain, skin and heart. Hyperplasia of endothelial cells and localized thrombus formation lead to obstruction of blood flow, with escape of RBC's into the surrounding tissue. Inflammatory cells also accumulate about affected segments of blood vessels. This angiitis appears to account for some of the more prominent clinical manifestations, such as petechial rash, stupor and terminal shock. Death is ascribed to damage of endothelial cells, resulting in leakage of plasma, decrease in blood volume, and shock.
It is assumed that the observed clinical manifestations of a rickettsial infection are due to production of an endotoxin, although this endotoxin is quite different in physiological effects from that produced by members of the Enterobacteriaceae. This is inferred, although the toxin has not been isolated, from these facts:
2. UV-irradiation of rickettsia diminished their infectivity without reducing toxicity.
3. The use of anti-rickettsial drugs does not prevent rapid death in experimental animals.
4. Antiserum specific for cell wall antigens of the rickettsia prevents the toxic effect.
Presumptive laboratory diagnosis is based on the finding of rickettsial-like organisms in tissue or blood. Although the organisms are gram-negative, they only weakly take the counter stain, safranin. Therefore, special staining procedures are used. Infected tissue may be stained with:
2. Castaneda stain--blue organisms against a red background.
3. Giemsa stain--bluish purple organisms.
Diseases
The
rickettsial diseases of man are usually broken down according to the arthropod
vector as seen in table below.
|
|
|
|
|
| 1. Louse-borne | |||
| European epidemic typhus | Rickettsia prowazekii | ------------------------------------------- | OX-19 |
| Brill's disease | Rickettsia prowazekii | ------------------------------------------- | Negative |
| Trench fever | Bartonella quintana | ------------------------------------------- | Negative |
| 2. Flea-borne | |||
| Endemic murine typhus | Rickettsia typhi | Wild rodents | OX-19 |
|
Cat scratch
fever/Bacilliary angiomatosis |
Bartonella henselae | Domestic cat | Unknown |
| 3. Mite-borne | |||
| Scrub typhus | Orientia (Rickettsia) tsutsugamushi | Wild rodents | OX-K |
| Rickettsialpox | Rickettsia akari | House mice | Negative |
| 4. Tick-borne | |||
|
Rocky Mountain Spotted
Fever |
Rickettsia rickettsii | Dog, rodents | OX-19, OX-2 |
| North Asian tick typhus | Rickettsia siberica | Wild rodents | OX-19, OX-2 |
| Fievre boutonneuse | Rickettsia conorii | Dog, rodents | OX-19, OX-2 |
| Queensland tick typhus | Rickettsia australis | Marsupials, rodents | OX-19, OX-2 |
| Q-fever | Coxiella burnetii | Cattle, sheep, goats | Negative |
| Spotted fever | Rickettsia rhipicephali | Dogs | Unknown |
| Ehrlichiosis | Ehrlichia canis | Dogs | Negative |
| Ehrlichiosis | Ehrlichia chaffeensis | Dogs | Negative |
| 5. Fly-borne | |||
| Oroyo fever/Verruga peruana | Bartonella bacilliformis | Unknown | |
Chemotherapy
The drugs of choice for the treatment of rickettsial diseases are chloramphenicol and tetracycline. Each of these is highly toxic, especially in children, and must be used with care. The sulfonamides stimulate rickettsial growth and thus are contraindicated in the treatment of these diseases.
General Characteristics
The chlamydia, which are incorrectly called the PLT viruses or Bedsonia or basophilic viruses, are bacteria which are obligate intracellular parasites of higher animals (mammals and birds). The members of this group share a unique development cycle, a common morphology and a common family antigen. They are not transmitted by arthropods. These organisms are termed basophilic because they take up the Giemsa stain (i.e., they stain blue). They are gram-negative, non-motile and multiply in the cytoplasm of the host cell. These organisms generally parasitize epithelial cells. The methods used to study them are, in the main, those of the virologist rather than the bacteriologist. Furthermore, the clinical features, pathogenesis, pathology and epidemiology of chlamydial infections are similar to those of viral infections.
Taxonomy
The chlamydia fall into two main ecological groups. In the first group, are the agents causing trachoma, inclusion conjunctivitis, and lymphogranuloma venereum, which seem to infect man only. In the second group, are those agents transmitted to man as zoonotic infections. About 100 species of birds are naturally infected with chlamydia. This includes 71 species of parrots as well as finches, pigeons, chickens, ducks, turkeys and seabirds. The chlamydia are thought to have evolved in the following way:
Subgroup A Subgroup B
Mammalian parasites
Primarily bird parasites
Compact inclusions Diffuse inclusions
Glycogen synthesized Glycogen not synthesized
Folates synthesized Folates not synthesized
Sensitive to D-cycloserine Resistant to D-cycloserine
Restricted host range Broadening of host range
Chlamydia trachomatis Chlamydia psittaci
Seven strains which are probably
Ten strains which are probably
distinct species
distinct species
Chlamydia pneumoniae
Only one serotype has been identified
Morphology and Structure
The chlamydial cell is roughly spherical and measures between 0.3 and 1.0 u in diameter, according to the stage of development. Both the small and the large cell types contain complete cell walls which are similar to the cell walls of gram-negative bacteria.
Under the cell wall lies a separate cytoplasmic membrane made up of large amounts of lipid. The DNA occurs as an irregular mass in the cytoplasm. There is no nuclear membrane. Ribosomes can be seen throughout the cytoplasm. The cells contain no capsule or flagella.
Metabolism
There are no detectable flavoproteins or cytochromes. It appears that the basis of the obligate intracellular parasitism is due to a lack of ATP-generating ability and the need to obtain ATP from the host cell. The cells can synthesize DNA, RNA and protein.
Growth and multiplication
Chlamydia pass through a series of developmental forms while multiplying by binary fission. This is termed the "developmental cycle." Two morphologically different developmental forms with a continuous gradation of intermediates between them can be recognized. One is a small cell about 0.3 u in diameter, with an electron-dense nucleoid. The other is a large cell, 0.5 to 1.0 u in diameter without a dense center.
There appears to be no significant difference in morphology or developmental cycle among the various chlamydia, and a single generalized description applies to all. The development cycle may be regarded as an orderly alternation of the small and large cell type. It is initiated by the highly infectious small cell which is taken into the host cell by phagocytosis. The engulfed small cell retains its morphological integrity in vacuoles bound by membrane derived from the surface of the host cell, and there is no eclipse (period in which the parasite loses the infectious ability). Instead, without loss of individuality, the small cell is reorganized into a large cell which is the vegetative multiplying form of these organisms. Then, still within the membrane-bound vacuole, the large cell grows in size and multiplies by repeated binary fission. The developmental cycle is completed by the reorganization of most of the large cells into small ones which are then available for infection of new host cells. The time required for completion of a cycle varies from 24-48 hours, depending on the particular host/parasite system involved.
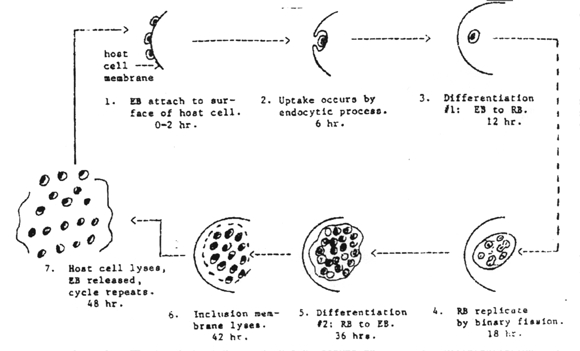
Characteristics
of the elementary and reticulate bodies of Chlamydia can be found in the
table below.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pathogenicity
Subgroup A organisms primarily infect the mucous membranes of the eye or the genitourinary tract of humans. Subgroup B organisms, although primarily parasites of birds, can be transmitted to man where they cause a lung infection.
The mechanism by which chlamydia cause disease or injure cells is unknown. Chlamydial infections of mucous membranes cause damage to tissues deep in the epithelial layer; for example, in trachoma, scarring of the tarsal plate occurs frequently. There is some evidence that a toxin is produced.
Laboratory Diagnosis
Laboratory diagnosis is made by one or more of the following:
1.
Isolation of the organism from infected tissue. The tissue is inoculated
into the yolk sac of seven-day chick embryos
or in McCoy human cells.
2. Characteristic cytoplasmic inclusion bodies infected cells.
3. Serological diagnosis:
a. Microimmunofluorescent tests in tears of patients
with eye infections for the presence of anti-chlamydia
antibody. In neonatal conjunctivitis and early trachoma, direct immunofluorescence
of conjunctive cells with
fluorescein - conjugated monoclonal antibody is sensitive and specific.
b. Delayed-type skin reaction (type IV hypersensitivity) to killed organisms in genitourinary infections (Frei test).
c. Rising titer of antibody against the chlamydial family
antigen in lung infecitons. This accomplished with the
complement fixation test or the fluorescent antibody test.
Treatment
Chlamydia exhibit low pathogenicity except in a compromised host. The chlamydial diseases are relatively easy to treat, but present two problems.
2.
Susceptibility of compromised host to reinfection--the compromised host
usually remains compromised
because of genetic and/or environmental factors and becomes reinfected.
3. Minimal symptomology
Chlamydia
trachomatis - doxycycline or azithromycin
Chlamydia
pneumonia - doxycycline or azithromycin or erythromycin
Chlamydia
psittaci - doxycycline or erythromycin
The
chlamydial diseases include:
| DISEASE | CAUSUAL AGENT | HOST |
| Subgroup A (person-to-person transmition) | ||
| Trachoma | Chlamydia trachomatis | Man |
| Inclusion conjunctivitis | Chlamydia trachomatis | Fowl, Man |
| Urethritis | Chlamydia trachomatis | Man |
| Cervicitis | Chlamydia trachomatis | Man |
| Ophthalmia neonatorum | Chlamydia trachomatis | Man |
| Myocarditis | Chlamydia trachomatis | Man |
| Lymphogranuloma venereum | Chlamydia trachomatis | Man |
| Atherosclerosis | Chlamydia trachomatis | Man |
| Neonatal Pneumonia | Chlamydia trachomatis | Man |
| -------------------------------------------------------------- | -------------------------------------------------------------------------------- | ------------------------------------------------ |
| Subgroup
B (mostly bird-to-human
(transmition) |
||
| Bronchitis/pneumonia/sinusitis | Chlamydia pneumoniae | Man |
| Atherosclerosis | Chlamydia pneumoniae | Man |
| Meningopneumonitis | Chlamydia psittaci | Birds --> Man |
| Hepatic and renal dysfunction | Chlamydia psttaci | Birds --> Man |
| Conjunctivitis | Chlamydia psttaci | Birds --> Man |
| Abortion | Chlamydia psttaci | Birds --> Man |
| Endocarditis | Chlamydia psttaci | Birds --> Man |
Chlamydia
has on its surface, a peptide that resembles one in heart myosin.
The peptide, when displayed by antigen-presenting cells, can trigger T-cells
that attack both Chlamydia and heart cells, thus causing heart muscle
inflammation (myocarditis). This autoimmune reaction also plays a
role in the formation of the artery-clogging plaques of atherosclerosis.
General Characteristics
The mycoplasmas are essentially bacteria lacking a rigid cell wall during their entire life cycle, although they are also much smaller than bacteria. The first organism of this type was associated with pleuropneumonia of cattle, and was originally called the pleuropneumonia organism (PPO). Since that time, a number of organisms with similar morphological characteristics and cultural properties have been isolated. These are commonly referred to as pleuropneumonia-like organisms or PPLO. A certain group of mycoplasmas produce extremely tiny colonies on agar plates, and are called the T-strains.
Some bacteria readily give rise spontaneously to variants that can replicate in the form of small filterable protoplasmic elements with defective or absent cell walls. These organisms, called L-forms, can also be formed by many species when cell wall synthesis is impaired by antibiotic treatment or high salt concentration. These organisms differ from mycoplasma in that they contain a rigid cell wall, at least at one stage of their life cycle and contain no sterols in their cytoplasmic membrane.
These organisms are the smallest known free-living organisms. Because of the absence of cell walls, they do not stain with the Gram stain, and they are more pleomorphic and plastic than eubacteria. With Giemsa stain, they appear as tiny pleomorphic cocci, short rods, short spirals, and sometimes as hollow ring forms. Their diameter ranges from 0.15 u to 0.30 u.
Most mycoplasmas require a rich medium containing a sterol and serum proteins for growth. Despite the lack of a cell wall, they do not require a medium of very high osmotic pressure. On solid media, they form minute, transparent colonies. When viewed under low-power magnification, the colony looks like a fried egg. The different strains vary in their growth rate and may take from two days to several weeks to form a colony.
Structure
The cell is enclosed by a limiting membrane which is more similar to that of animal cells than that of bacterial cells because of sterols present in the membrane. The cytoplasm contains ribosomes, but lacks mesosomes. There is no nuclear membrane. In some strains, amorphous material on the outer surface of the membrane suggests the existence of a capsule.
Metabolism
The
parasitic mycoplasmas have truncated respiratory systems, lacking quinones
and cytochromes. Another indication for the simplicity of the electron
transport chain is the finding that the reduced nicotinamide adenine dinucleotide
(NADH) oxidase activity is cytoplasmic. Complex electron transport chains
are usually membrane bound, since they depend on the spatial organization
of their components. Ruling out oxidative phosphorylation as an ATP-generating
system leaves only two proven ways of ATP generation, both based on substrate
level phosphorylation. The major source for ATP is the arginine dihydrolase
pathway.
arginine deaminase
Arginine
+ H2O ----------------------------> citrulline + NH3
ornithine carbamoyltransferase
Citrulline+inorganic
orthophosphate-------------------------------------------->ornithine+carbamoylPO
carbamate kinase
Carbamoyl
PO4 + ADP --------------------------> ATP + CO2 +
NH3
Another mechanism for ATP generation is:
phosphate acetyltransferase
Acetyl
CoA + inorganic orthophosphate ----------------------------------------->
acetyl PO4 + CoA
acetate kinase
Acetyl
PO4+ ADP ------------------------------------> Acetate + ATP
Acetyl CoA is produced by oxidative decarboxylation of pyruvate.
A few species derive their energy from the degradation of glucose or the hydrolysis of urea. All species synthesize DNA, RNA, lipids and proteins. However, it is not known if they can synthesize amino acids. Those species that require sterols incorporate these sterols (mainly cholesterol) into the cell membrane up to concentrations of 65%.
Multiplication
In the absence of a rigid cell wall, the pattern of replication is quite different from that of typical bacteria, whose division starts with the formation of a well-defined septum. Though the mechanism of division in mycoplasmas is controversial, sequential microscopic observation suggests that new elementary particles arise by fragmentation of filamentous cells containing several discrete DNA components.
Pathogenesis
M. pneumoniae is an extracellular pathogen that adheres to the respiratory epithelium by a specialized terminal protein attachment factor. This adherence protein interacts specifically with neuraminic acid residues on the epithelial cell surface. Ciliastasis occurs following attachment and then destruction of the superficial layer of epithelial cells. Destruction is due to release of hydrogen peroxide and superoxide anion.
Diseases
The
human diseases caused by mycoplasmas are shown in the table below.
| DISEASE OR SYMPTOM | AGENT | HOST |
| Primary atypical pneumonia | Mycoplasma pneumoniae | Man |
| Non-gonococcal urethritis (NGU) | Mycoplasma genitalium | Man |
| NGU | Ureaplasma urealyticum | Man |
| Stillbirth | Mycoplasma hominis | Man |
| Spontaneous abortion | Mycoplasma hominis | Man |
| Infertility | Mycoplasma hominis | Man |
Laboratory Diagnosis
The laboratory diagnosis of mycoplasma infection can be accomplished by:
1.
Culturing the organism from sputum, mucous membrane swabbings or other
specimens by direct inoculation into
liquid or solid media containing serum, yeast extract and penicillin to
inhibit contaminating bacteria. Colonies will
become detectable in one to three weeks. They stain intensely with neutral
red or tetrazolium or methylene blue.
The organism can be presumptively identified by its hemabsorption or B-hemolysis
of guinea pig red blood cells. It
is conclusively identified by staining its colonies with homologous fluorescein-labelled
antibody.
2.
Quantitation of the patient antibody response to mycoplasma by complement
fixation tests on acute and
convalescent serum. Cold agglutinins to human O erythrocytes may
also be measured.
Chemotherapy
Primary atypical pneumonia is usually selflimiting and does not require antibiotic treatment. However, if antibiotics are needed, the drug of choice is one of the macrolide antibiotics:
Azithromycin
Clarithromycin
Dirithromycin
Erythromycin
Urogenital diseases may be treated with:
Metronidazole (except during the first trimester of pregnancy)
Clindamycin
1.
The rickettsia are extremely small gram-negative rod-shaped, coccoid or
pleomorphic bacteria with limited
metabolic capabilities.
2. The Family Rickettsiaceae contains three genera: Rickettsia, Ehrlichia, and Coxiella.
3.
All of the members of the Family Rickettsiaceae are obligate intracellular
parasites due to a highly permeable
cytoplasmic membrane.
4.
Confirmative diagnosis of rickettsial diseases is accomplished via the
Weil-Felix test, a complement fixation test
and/or an indirect fluorescent antibody test.
5. The drugs of choice for the treatment of rickettsial diseases are chloramphenicol and tetracycline.
6.
Louse-borne rickettsial diseases include European epidemic typhus, and
Brill's disease. Man is the sole reservoir for
louse-borne diseases.
7. The only flea-borne rickettsial disease is endemic murine typhus.
8. Mite-borne rickettsial diseases include scrub typhus and rickettsialpox.
9.
Tick-borne rickettsial diseases include Rocky Mountain spotted fever, North
Asian tick typhus, Fievre boutonneuse,
Queensland tick typhus, Q fever, spotted fever and ehrlichiosis.
10. The major target tissue of the rickettsia is vascular endothelium.
11.
The chlamydia are extremely small, gram-negative, coccal-shaped, obligate
intracellular parasites with limited
metabolic capability which are classified as bacteria.
12. The chlamydia lack flavoproteins and cytochromes.
13.
Chlamydia undergo a unique developmental cycle which is an alternation
in size between the small elementary
body and the relatively large reticulate body.
14.
The elementary body is relatively metabolically inactive, adapted for extracellular
survival and is the infectious
unit.
15. The reticulate body is metabolically active, adapted for intracellular growth and is not infectious.
16. The major target tissue of the chlamydia is mucous membranes.
17.
Serological diagnosis of chlamydial diseases is via a fluorescent antibody
test, complement fixation test or a
delayed type hypersensitivity test (Frei test) for lymphogranuloma venereum.
18.
Chlamydial diseases include trachoma, inclusion conjunctivitis, lymphogranuloma
venereum, bronchitis,
pneumonia, sinusitis and meningopneumonitis, hepatic and renal dysfunction,
abortion and endocarditis.
19. The mycoplasma are extremely small free-living bacteria which lack a cell wall and cytochromes.
20. Mycoplasma can be cultured on agar media but colonies take up to three weeks to develop.
21.
Serological identification of mycoplasma disease relies upon the quantitation
of cold agglutinins to human O
erythrocytes or a complement fixation test or serum inhibition of mycoplasma
growth.
22.
Human diseases of mycoplasma etiology are primary atypical pneumonia, non-gonococcal
urethritis, stillbirth,
spontaneous abortion and infertility.