A severe and sometimes chronic form of viral hepatitis, known as blood-borne viral hepatitis, is transmitted via blood transfusions, blood products, needle sticks, shared drug paraphernalia, breast-feeding, and sexual contact.
Etiology
There are three different viruses that infect the liver and result in blood-borne viral hepatitis: hepatitis B virus (HBV), hepatitis C virus (HCV), and hepatitis D virus (HDV). HBV is an enveloped partially double-strand DNA virus in the hepadnavirus family. HCV is an enveloped single-strand RNA virus in the flavivirus family. HCV has 6 different genotypes (i.e., 1-6) and many different subtypes (e.g., 1a, 1b, 2a, 2b). Response to treatment depends on the HCV genotype the patient is infected with. HDV is a single-strand RNA viroid called the delta agent. The HDV agent is a defective virus that cannot infect a hepatocyte without the hepatocyte also being infected with HBV. To infect a hepatocyte, HDV must be coated with HBsAg. HBV and HDV can coinfect a person simultaneously (coinfection) or HDV can infect a patient after infection with HBV (superinfection). Thus, delta agent is found only in concurrent HBV infections.
Manifestations
HBV
The incubation period for HBV infection is longer than that for HAV infection and is anywhere from 7 to 160 days in duration. Asymptomatic infections occur about 65% of the time. Most children do not have any symptoms of HBV infection; however, when symptoms occur in children, they are usually less severe than symptoms in adults. Early symptoms of acute infection include fatigue, anorexia, nausea, pain and fullness in the upper right quadrant, and fever. Manifestations that develop later may include arthritis, rash, and cholestasis (jaundice and icterus), and tend to be more severe than in patients infected with HAV.
Fulminant HBV hepatitis occurs in 1% of acutely infected individuals. Fulminant hepatitis results in altered brain function (hepatic encephalopathy), extensive jaundice, and massive necrosis of the liver. Manifestations are more severe and can be fatal. Patients have severe liver damage with abdominal ascites and bleeding; the liver shrinks rather than swells.
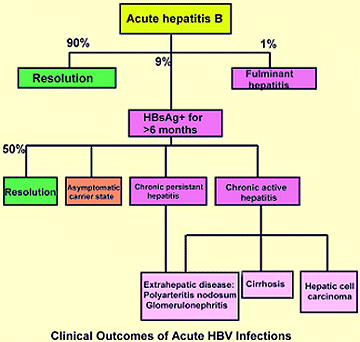
Chronic HBV hepatitis occurs in 5-10% of patients and is more likely to occur in those that had a mild or inapparent initial infection. Chronic HBV hepatitis is defined as the presence of HBV surface antigen in the bloodstream for at least 6 months. Around 50% of those with chronic HBV hepatitis will resolve the viral infection of the liver with no long term sequelae. Approximately, 20% of those with chronic HBV hepatitis will become asymptomatic chronic carriers of the virus or chronic persistent hepatitis patients and they may experience extrahepatic disease (e.g., glomerulonephritis, polyarteritis nodosum). The remaining 30% of chronic HBV patients develop chronic active hepatitis and may develop cirrhosis of the liver, primary hepatocellular carcinoma, or extrahepatic disease. See diagnosis section to determine whether a patient has chronic active or chronic persistent HBV hepatitis.

HCV
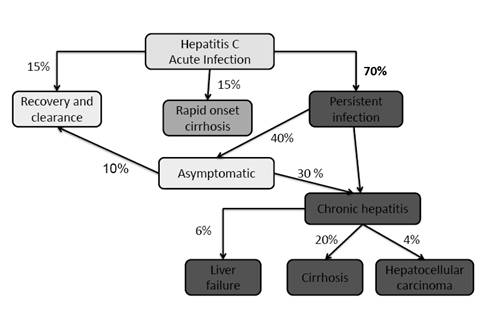
Around 15% of patients with HCV infection will develop symptomatic acute hepatitis after an incubation period of 14-180 days and then fully recover without developing chronic hepatitis. The acute form of HCV hepatitis is similar to HBV but the inflammatory response is less and as a result symptoms are usually milder.
Approximately, 70% of those infected with HCV will be asymptomatic and they will become chronic persistent hepatitis patients. Chronic persistent HCV hepatitis is defined as the presence of HCV RNA in the bloodstream for at least 6 months. The predominant symptom, if any, is chronic fatigue. Chronic persistent HCV hepatitis patients oftentimes progress to chronic active HCV hepatitis in 10-15 years leading to cirrhosis (20% of the cases) and liver failure (20% of cirrhotic cases) after 20 years. Alcohol consumption, certain medications, and other hepatitis viruses can exacerbate HCV-induced liver damage to promote cirrhosis of the liver. HCV chronic hepatitis infections results in primary hepatocellular carcinoma in around 5% of these patients after around 30 years.
Lastly, around 15% of those infected with HCV develop rapid onset cirrhosis of the liver.
HDV
HDV requires the presence of HBV to infect the liver. HDV increases the severity of HBV infections. Superinfection is more likely to result in more severe disease than coinfection. Patients with HBV/HDV infections can experience acute hepatitis, fulminant hepatitis, cirrhosis, or chronic HDV infections. Fulminant hepatitis is more likely to occur in patients infected with HDV than those infected with the other hepatitis viruses. Around 80% of patients with fulminant hepatitis die.
Chronic Hepatitis
Chronic infections are not seen in patients infected with HAV and HEV, but are observed in patients with HBV, HCV and HDV infections. Chronic hepatitis usually follows mild or asymptomatic initial infection. Most chronically infected patients are asymptomatic for many years; however, in time, liver damage can result in cirrhosis and liver failure. Chronic viral hepatitis is usually detected by elevated liver enzyme levels (AST and ALT) obtained on a routine blood analysis.
Primary hepatocellular carcinoma (HCC) can occur in patients with chronic HBV or HCV infection. HCC can develop in a patient who has been infected with HBV or HCV anywhere from 9 to 35 years later.
Epidemiology
HBV, HCV, and HDV
HBV
Table HEP-3. Persons at High Risk for Hepatitis B (HBV) Infection |
|
HCV
HDV
HBV
HBV enters the bloodstream and infects the cells of the liver by replicating within the cells. Symptoms may not be observed for 45 days or more, depending on the dose of HBV, the route of infection, and the individual. HBV genomes integrate into host chromosomes during replication and are the basis of chronic infections. Large amounts of HBV surface antigen (HBsAg) and virions are released in the blood.
Immune complexes formed by HBsAg and antibody are responsible for hypersensitivity reactions that occur in some patients (e.g., arthritis, rash, liver damage, vasculitis, or arthralgia). Liver parenchymal cell degeneration results from cellular swelling and necrosis; resolution of the infection allows the liver parenchyma to regenerate. Fulminant HBV infection, activation of chronic infection, or coinfection with the delta agent can lead to permanent liver damage or cirrhosis. Both cell-mediated immunity and inflammation are responsible for the resolution of HBV infection and its symptoms. Acute cases of HBV disease are usually of short duration with significant symptomology. Figure VH-1 (see below) shows a graphic representation of the pathogenesis of an acute HBV infection.
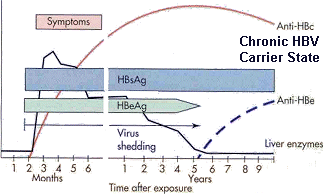
Chronic HBV infection is defined as the presence of HBsAg in the bloodstream following infection by HBV for at least 6 months. The early phase of chronic HBV infection is characterized by the presence of HBeAg and high serum levels of HBV DNA (referred to as HBeAg-positive chronic HBV) (Figure VH-2 see below).
During a chronic HBV infection, the immune system attempts to clear the HBV by destroying infected hepatocytes, which leads to increasing circulatory blood levels of ALT. Most patients will clear HBeAg (hepatitis B envelope antigen) from the bloodstream, produce antibodies to HBeAg (anti-HBe), and achieve a state of nonreplicative infection that is characterized by low or undetectable serum levels of HBV DNA and normal ALT levels.
Over time, 25% of persons who acquire HBV as children will have a chronic HBV infection that results in cirrhosis or HCC as adults. Cirrhosis may develop as a consequence of repeated immune system attacks in which the normal hepatocytes are destroyed and replaced with fibrous tissue. Once established, cirrhosis cannot be cured; however, its progress may be stopped if the cause is removed. Without treatment, the typical progression is from compensated cirrhosis to decompensated cirrhosis. Decompensated cirrhosis is characterized by cessation of enzymatic processes in the liver and subsequent severe clinical complications such as fluid retention in the abdomen (ascites), jaundice, internal bleeding, and hepatic encephalopathy. Patients with decompensated cirrhosis are candidates for liver transplantation; without transplantation, death results from end-stage liver disease.
HBV carcinogenesis
The carcinogenesis of hepatocellular carcinoma (HCC) is a multi-step process involving a number of different genetic alterations that result in malignant transformation of the hepatocyte. Chronic HBV infection appears to cause HCC by direct and indirect mechanisms. Continuous hepatocyte injury and regeneration in cirrhosis of the liver due to HBV replication leads to increased hepatocyte turnover and accumulation of critical mutations in the genome of these cells. These mutations may cause chromosomal rearrangements as well as activation of cellular oncogenes and inactivation of tumour suppressor genes.
A higher rate of chromosomal abnormalities is found in HBV-related HCC than can be explained by the accumulation of chromosomal mutations alone. HBV is an oncogenic virus and can integrate its DNA into the genome of hepatocytes. Integrated HBV DNA sequences are present in about 80% of human HBV related HCCs. HBV DNA integration may confer a selective growth advantage on infected hepatocytes and leads to the onset of tumor progression. The integration sites of HBV DNA usually occur in cellular genes involving cell signaling or growth control. When HBV DNA integrates into the host chromosome chromosomal instability is also increased (e.g., large inverted duplications, deletions, amplification, and chromosomal translocation).
HCV
Not much is known about the pathogenesis of HCV infection. We do know that after HCV infects hepatocytes it can remain cell associated and prevent death of the host cell it infects. This appears to protect the virus from elimination from the host and makes it more likely to cause chronic infections and liver damage years after the initial infection. Cell-mediated immune responses appear to cause most of the liver damage. The appearance of a vigorous and multispecific CD4+ and CD8+ T cell response in the blood of persons with acute HCV infection appears to indicate the patient will recover from the infection. In contrast, a lack or waning of the T cell responses seems to indicate the patient will develop chronic HCV disease.
HCV carcinogenesis
The longer the chronic HCV infection and the larger the area of liver fibrosis the more likely a person will develop HCC. The risk of developing HCC for cirrhotic HCV patients was the highest, compared to those who had less liver fibrosis.
HCV does not have a reverse transcriptase so unlike HBV it is not able to integrate into the genome of hepatocytes. HCV causes HCC by an indirect pathway when it produces chronic inflammation, cell death, proliferation and cirrhosis in the liver. As a result, HCV-induced HCCs are almost always found in patients with cirrhosis. HCCs without cirrhosis in HCV-infected patients, though rare, have been reported. HCV NS3 protein and core proteins are believed to be involved in carcinogenesis in patients with little or no liver cirrhosis.
HDV
HDV can only replicate in people with active HBV infections. Since HDV and HBV are transmitted by the same routes a person can be coinfected with HDV and HBV by acquiring both viruses at the same time or a chronic HBV patient can acquire the HDV virus and become superinfected. Patients that are superinfected usually experience more rapid and severe liver damage. HDV in coinfected patients must wait for HBV to establish infection in the liver before it can cause liver damage however, in patients with chronic HBV infections HDV can start replicating right away and speed the progression of the infection in the liver. Replication of the HDV agent causes cytotoxicity and liver damage. Pathology in the liver is a direct result of the cytopathic effects of HDV replication. Protection from HDV infections is not due to antibody to HDV but rather due to antibodies reactive with HBV surface antigen. Therefore, immunizing a patient to protect them from HBV infection also protects them from getting HDV infections.
Diagnosis
Initial diagnosis of HBV, HCV, and HDV infections is usually determined clinically if signs and symptoms are present. Evaluation of the patient’s blood for elevations in ALT, AST, alkaline phosphatase, and bilirubin aids in making an initial diagnosis. A confirmatory diagnosis can be determined following reactivity in various serologic tests for HBV (Table HEP-4; hepatitis panel for serologies), HCV, and HDV.
In patients with acute disease tests to detect HCV RNA are usually most sensitive and will be positive before serological tests. Testing an HCV RNA positive patient’s blood for antibodies 5-7 days later confirms HCV infection when the tests are positive and it is highly likely the patient is experiencing acute hepatitis. However, if both the RNA and antibody tests are positive it is difficult to determine if this is an acute or chronic HCV hepatitis and the signs and symptoms of hepatitis are due to some other cause.
Acute HBV Infection Assays
Blood tests for HBV infections are more complicated than most serologic tests. Tests for HBV infection detect both viral antigens and antibodies to viral antigens. To confirm whether a patient has HBV infection, three blood tests are usually ordered: HBV surface antigen (HBsAg), antibody to HBV core antigen (anti-HBcAg), and antibody to HBsAg (anti-HBsAg) (Table HEP-4).
During the acute phase of HBV infection, the virus is actively dividing and infectious and incomplete viral particles are being produced. These infectious and incomplete viral particles enter the bloodstream with enough HBsAg present in the blood to be detected in samples. Patients who are positive for HBsAg have early acute HBV infection (Table HEP-4; Figure VH-1).
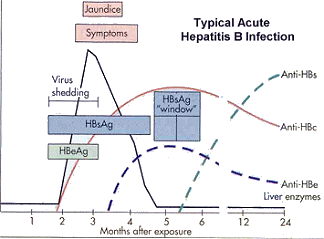
Figure VH-1
Within 4–5 weeks following HBV infection, patients who recover from the infection will produce antibodies to HBsAg and HBcAg. The anti-HBsAg produced by the patient removes the HBsAg from the bloodstream. HBV infected patients become negative for both HBsAg and anti-HBsAg, making diagnosis of HBV infection impossible if only these two molecules are assayed. Fortunately, IgM antibody to HBcAg (IgM anti-HBcAg) continues to be detectable in the bloodstream. The period when HBsAg and anti-HBsAg are no longer detectable but IgM anti-HBcAg is detectable is called the “window period” (Table HEP-4; Figure VH-1).
Within 5–6 weeks following infection, as virus replication is stopped by the immune response, enough HBsAg is eliminated from the blood and anti-HBsAg is detectable. By this time, patients will also be producing IgG to HBcAg (IgG anti-HBcAg). A patient infected with HBV who is negative for HBsAg but positive for anti-HBsAg and IgG anti-HBcAg is entering convalescence (see Table HEP-4; Figure VH-1). Eventually, anti-HBsAg is not detectable but most persons with a previous HBV infection will be positive for IgG anti-HBcAg (Table HEP-4; Figure VH-1).
Chronic HBV Infection Assays
If a patient becomes chronically infected with HBV, HBsAg is not eliminated from the bloodstream and can be detected for long periods of time. By definition, any patient who has detectable HBsAg for 6 months or longer has a chronic HBV infection (Table HEP-4; Figure VH-2).
VH-2
An HBeAg test is used to determine if a patient infected with HBV is infectious; patients with anti-HBeAg are not very infectious. Patients who have chronic HBV hepatitis with HBeAg are infectious and usually have a much worse prognosis (chronic active hepatitis), whereas patients with chronic HBV infection and anti-HBeAg (chronic persistent hepatitis) are less likely to have serious infections and are not considered as infectious.
Table HEP-4. Hepatitis Panel for Hepatitis B (HBV) |
||||||
Stage of Hepatitis Infection |
HBsAg |
Anti-HBsAg |
Anti-HBcIgM |
Anti-HBc IgG |
HBeAg |
Anti- HBeAg |
Early acute HBV |
+ |
- |
+ |
- |
+ |
- |
“Window period” HBV |
- |
- |
+ |
- |
+/- |
+/- |
Convalescent HBV |
- |
+ |
- |
+ |
- |
+ |
Late convalescent HBV |
- |
- |
- |
+ |
- |
+ |
Chronic active hepatitis |
Positive for > 6 months |
- |
- |
+ |
+ |
- |
Chronic persistent hepatitis |
Positive for > 6 months |
- |
- |
+ |
- |
+ |
Vaccinated for HBV |
- |
+ |
- |
- |
- |
- |
HBsAg, hepatitis B virus surface antigen; anti-HBsAg, antibody to HBsAg; anti-HBc IgM, immunoglobulin M antibody to HBV core antigen; anti-HBc IgG, immunoglobulin G antibody to HBV core antigen; HBeAg, HBV envelope antigen; anti-HBeAg, antibody to HBeAg
Therapy and Prevention
Treatment of acute infections with HBV, HCV, and HDV involves supportive care. The goal of treatment for patients with chronic HBV and HCV is to get a sustained virologic response or SVR. SVR occurs when undetectable serum levels of HBV DNA or HCV RNA results following treatment for a specified period of time.
Treatment is recommended for patients with evidence of chronic active hepatitis B disease (i.e., abnormal aminotransferase levels, positive HBV DNA findings, positive or negative hepatitis B e antigen [HBeAg]). Pegylated interferon alfa (PEG-IFN-a), entecavir (ETV), and tenofovir disoproxil fumarate (TDF) are the first-line agents in the treatment of HBV disease.
Two different treatment strategies exist for treating chronic HCV patients: (1) immediate treatment with anti-HCV medications for up to 24 weeks; or (2) watchful waiting with liver biopsy performed every 3 years and drug therapy initiated if hepatitis progresses to a moderate stage of liver damage.
HCV Treatment Recommendations |
||
Recommended* |
Alternative |
|
Genotype 1a |
Sofosbuvir/ledipasvir tablet for 12 weeks** OR Elbasvir and grazoprevir (Zepatier) for 12 weeks |
n/a |
Genotype 1b |
Sofosbuvir/ledipasvir tablet for 12 weeks** OR Elbasvir and grazoprevir (Zepatier) for 12 weeks |
n/a |
Genotype 2 |
Sofosbuvir + ribavirin for 12 weeks |
none |
Genotype 3 |
Sofosbuvir + ribavirin for 24 weeks |
Sofosbuvir + ribavirin + PEG*** for 12 weeks |
Genotype 4 |
Sofosbuvir/ledipasvir tablet for 12 weeks OR Elbasvir and grazoprevir (Zepatier) for 12 weeks |
Simeprevir + Sofosbuvir with/without |
Genotype 5 |
Sofosbuvir + ribavirin + PEG*** for 12 weeks |
PEG*** + ribavirin + for 48 weeks |
Genotype 6 |
Sofosbuvir/ledipasvir tablet for 12 weeks |
Sofosbuvir + ribavirin + PEG*** for 12 weeks |
* When more than one treatment is recommended, they are listed alphabetically
** 8 weeks may be considered in patients without cirrhosis who have pre-treatment HCV RNA less than 6 million IU/mL
*** PEG = peginterferon
**** Ombitasvir, paritaprevir and ritonavir tablets co-packaged with dasabuvir tablets= Viekira Pak
The type of treatment and the viral response to treatment depends on the genotype of HCV that has infected the patient. Those patients with genotypes 2 and 3 (70-80%) are more likely to have a sustained viral response (“cure”) (i.e., no detectable viral RNA in the blood 6 months after completion of therapy) than patients infection with genotype 1 (40-50%).
To prevent infection of both HBV and HDV, immunization with the HBV vaccine is recommended for all children. This vaccine was called the first “cancer vaccine” because of its ability to prevent chronic HBV infection and the resultant HCC that occurs in some chronically infected HBV patients. The vaccine contains purified recombinant HBsAg. Persons who have been successfully vaccinated will produce anti-HBs and may be confused as having had a prior HBV infection; however, persons who have been vaccinated do NOT produce anti-HBc. Persons who have had a prior HBV infection will produce anti-HBc; thus vaccinated persons can be differentiated from persons with a prior infection by assaying for antibody to both HBsAg and HBcAg.
HBV immunoglobulin prepared from plasma with a high titer of anti-HBsAg but no detectable HBsAg is used in combination with vaccination for infants born to HBV-positive mothers and for susceptible persons who were exposed to HBV.
Send comments and email to Neal R. Chamberlain, Ph.D., nchamberlain@atsu.edu
Revised 3/8/21
©2016 Neal R. Chamberlain, Ph.D., All rights reserved.